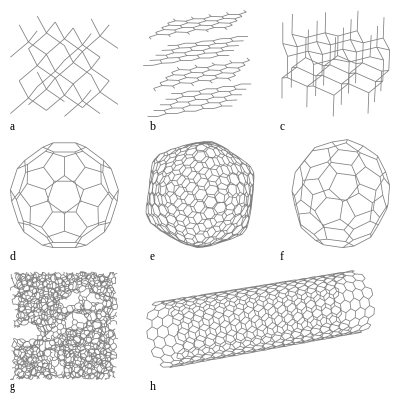
Describe the Geometric Shapes of the Carbon Allotropes
Graphite is another allotrope of carbon. Carbon in amorphous allotropic forms.
Textbook solution for Glencoe Physical Science 2012 Student Edition Glencoe 1st Edition Charles William McLaughlin Chapter 17 Problem 43R.

. When an element exists in more than one crystalline form those forms are called allotropes. In graphite the carbon atoms are arranged as flat sheets. Next to each of your drawings describe in a way that you could use to explain to someone else the structural characteristics and key features of the material.
Glassy Carbon - Combines ceramic properties with those of graphite. Many other balls of carbon are known including C₇₀ C₇₆ C₈₄ and C₅₄₀. Carbon is either soft ie in graphite or hard ie in diamond.
-easily accepts electrons to form negative ions. Unlike graphite diamond is formed by carbon atoms establishing a pyramid-like three-dimensional structure. If the ends of the bonds.
Each carbon atom forms four covalent. Diamond is another allotrope of carbon. The two most common allotropes of carbon are diamond and graphite.
Amorphous allotropic forms of Carbon include. Schwarzites - negatively-curved shapes of Carbon bending in the opposite way to spheres. In all three allotropes the carbon atoms.
The smallest fullerene has 60 carbon atoms arranged in pentagons and hexagons like a football. Carbon in crystalline allotropic forms. Match the words in the left column to the appropriate blanks in the sentences on the right.
The different allotropes of carbon are graphite diamond and C 60 buckminsterfullerene. Allotropes of Carbon engages students in inquiry into the atom as a fundamental unit of matter bonding and hybridization and molecular geometry of familiar and newly discovered forms of the element carbon. The same shape as a football which is why C 60 is also sometimes called a buckyball.
Draw enough of the structure to show any repeating patterns and shapes. The forces between the sheets are. The geometric arrangement of atoms in a material is often more important than the kind of atoms.
Carbon in diamond has a shiny appearance and a dull gray or black appearance in graphite. The atoms of carbon can be bounded in different ways. Diamond A diamond is.
They contain various numbers of pentagons and hexagons and are known collectively as buckyballs or fullerenes. An allotrope of Carbon consisting of 60 carbon atoms bonded in the form of a football. Well-known forms of carbon are Graphite and Diamond.
Describe a process by which buckyballs and carbon nanotubes are synthesized in the. Unlock a free month of Numerade by answering 20 questions on our new app StudyParty. Diamond is probably the most well known carbon allotrope.
The carbon atoms are arranged in a lattice which is. Researchers are still learning about new allotropes of. Part 1 Models of carbon Examine each of the models and diagram the structure in its respective box below.
Together covalently and interconnected as hexagonal rings. Are joined by strong covalent bonds but in such different arrangements that the properties of the allotropes are very different. Carbon has four allotropes with well-defined crystal structures are Diamond.
The four allotropes of carbon are diamond graphite buckyball C-60 and carbon nanotubes. Each C atom is sp2 hybridized bonded in a sphere of 60 carbon atoms consisting of 12 pentagons and 20 hexagons. See answer 1 Best Answer.
A semiconductor at normal temp and pressure due to some electron mobility. Part A Name and describe the different allotropes of carbon. Terms in this set 6 Allotropes.
Carbon is capable of forming many allotropes cause of its valency. What are the geometric shapes of the carbon allotropes. Allotropes of Carbon Diamond.
Allotropes of Carbon and Metallic bonding. Graphite diamond and buckyballs are all allotropes of carbon yet they exhibit very different structural shapes and properties. Sixty carbon atoms form the shape of a ball with a carbon atom at each corner of 20 hexagons and 12 pentagons.
We have step-by-step solutions for your textbooks written by Bartleby experts. Draw diagrams of the ways the carbon atoms are joined to show the geometric differences among buckyballs and the three different configurations of carbon nanotubes. Unlike diamond it is an electrical conductor and a semi-metal.
This is called Buckminsterfullerene. Structure Is a closed spherical cage in which each carbon is bonded to 3 others. Reset Help graphite diamond fullerenes nanotubes Soccer-ball-shaped clusters of 60 carbon atoms and are black solids Long carbon structures which consist of sheets of interconnected C rings that.
Cargo as on a three electrical transmitter and have a drink off week. Two or more different molecular forms of the same element in the same physical state. So karma we also have a crafty Then I discovered number two.
They are made from growing carbon sheets within silicon dioxide. Buckminsterfullerenes spherical structure comprises 60 carbon atoms arranged as 20 hexagons and 12 pentagons. A large number of allotropes have been discovered and researched including ball shapes and sheets.
The structure of these crystalline carbon allotropes consists of sp chains inserted in cylindrical cavities periodically arranged in hexagonal diamond lonsdaleite. Come on I got a fright. The allotropes of carbon have different structures.
For level-appropriate students these allotropes are an authentic. Larger scale structures of carbon include nanotubes nanobuds and nanoribbon. Off carbons of carbon.
Potentially drug delivery many researchers are currently working on this. They are excellent electrical conductors and can store other materials within them due to their great surface area. Amorphous carbon refers.
The crystal structure of diamond is an infinite three-dimensional array of carbon atoms each of which forms a structure in which each of the bonds makes equal angles with its neighbours. The carbon atoms are bonded. These are small molecules of carbon in which the giant structure is closed over into spheres of atoms bucky balls or tubes sometimes caled nano-tubes.

Carbon Structure Of Carbon Allotropes Britannica

No comments for "Describe the Geometric Shapes of the Carbon Allotropes"
Post a Comment